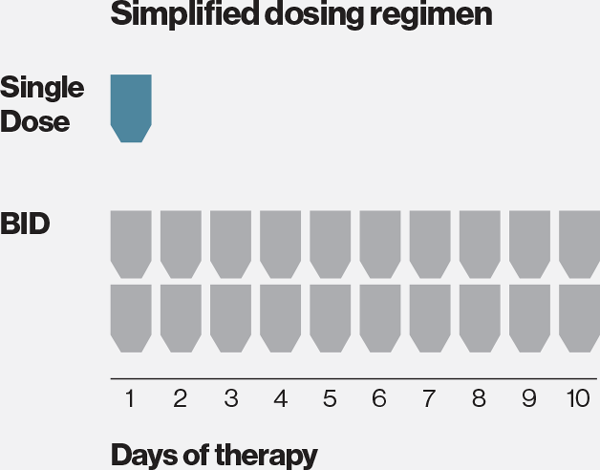
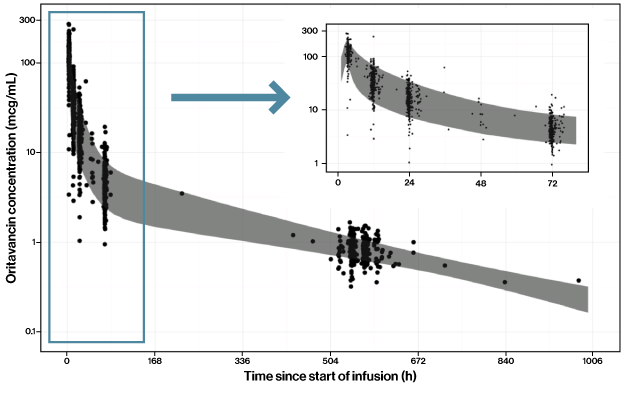
INDICATION AND USAGE
ORBACTIV® (oritavancin) for injection is indicated for the treatment of adult patients with acute bacterial skin and skin structure infections (ABSSSI) caused or suspected to be caused by susceptible isolates of the following gram-positive microorganisms: Staphylococcus aureus (including methicillin-susceptible [MSSA] and -resistant [MRSA] isolates), Streptococcus pyogenes, Streptococcus agalactiae, Streptococcus dysgalactiae, Streptococcus anginosus group (includes S. anginosus, S. intermedius, and S. constellatus), and Enterococcus faecalis (vancomycin-susceptible isolates only).
To reduce the development of drug-resistant bacteria and maintain the effectiveness of ORBACTIV® and other antibacterial drugs, ORBACTIV® should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria.
Important Safety Information
Contraindications
Use of intravenous unfractionated heparin sodium is contraindicated for 120 hours (5 days) after ORBACTIV® administration because the activated partial thromboplastin time (aPTT) test results may remain falsely elevated for approximately 120 hours (5 days) after ORBACTIV® administration.
ORBACTIV® is contraindicated in patients with known hypersensitivity to oritavancin products.
Warnings and Precautions
Coagulation test interference: Oritavancin has been shown to artificially prolong aPTT for up to 120 hours and may prolong PT and INR for up to 12 hours, and ACT for up to 24 hours. Oritavancin has also been shown to elevate D-dimer concentrations up to 72 hours. For patients who require aPTT monitoring within 120 hours of oritavancin dosing, consider a non-phospholipid dependent coagulation test such as a Factor Xa (chromogenic) assay or an alternative anticoagulant not requiring aPTT.
Serious hypersensitivity reactions, including anaphylaxis, have been reported with the use of oritavancin products, including ORBACTIV®. Discontinue infusion if signs of acute hypersensitivity occur. Closely monitor patients with known hypersensitivity to glycopeptides.
Infusion Related Reactions: Administer ORBACTIV® over 3 hours to minimize infusion-related reactions. Infusion reactions characterized by chest pain, back pain, chills and tremor have been observed with the use of oritavancin products (e.g. ORBACTIV®), including after the administration of more than one dose of oritavancin during a single course of therapy. Stopping or slowing the infusion may result in cessation of these reactions.
Clostridioides difficile-associated diarrhea: Evaluate patients if diarrhea occurs.
Concomitant warfarin use: Oritavancin has been shown to artificially prolong PT/INR for up to 12 hours. Patients should be monitored for bleeding if concomitantly receiving ORBACTIV® and warfarin.
Osteomyelitis: Institute appropriate alternate antibacterial therapy in patients with confirmed or suspected osteomyelitis.
Prescribing ORBACTIV® in the absence of a proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and increases the risk of development of drug-resistant bacteria.
Adverse Reactions
The most common adverse reactions (≥3%) in patients treated with ORBACTIV® were headache, nausea, vomiting, limb and subcutaneous abscesses, and diarrhea.
Please see Full Prescribing Information for ORBACTIV®.
INDICATION AND USAGE
ORBACTIV® (oritavancin) for injection is indicated for the treatment of adult patients with acute bacterial skin and skin structure infections (ABSSSI) caused or suspected to be caused by susceptible isolates of the following gram-positive microorganisms: Staphylococcus aureus (including methicillin-susceptible [MSSA] and -resistant [MRSA] isolates), Streptococcus pyogenes, Streptococcus agalactiae, Streptococcus dysgalactiae, Streptococcus anginosus group (includes S. anginosus, S. intermedius, and S. constellatus), and Enterococcus faecalis (vancomycin-susceptible isolates only).
To reduce the development of drug-resistant bacteria and maintain the effectiveness of ORBACTIV® and other antibacterial drugs, ORBACTIV® should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria.