Single dose ORBACTIV® (oritavancin) was effective at achieving early clinical response at 48-72 hours1-3
Primary endpoint: Early clinical response* rates at 48-72 hours
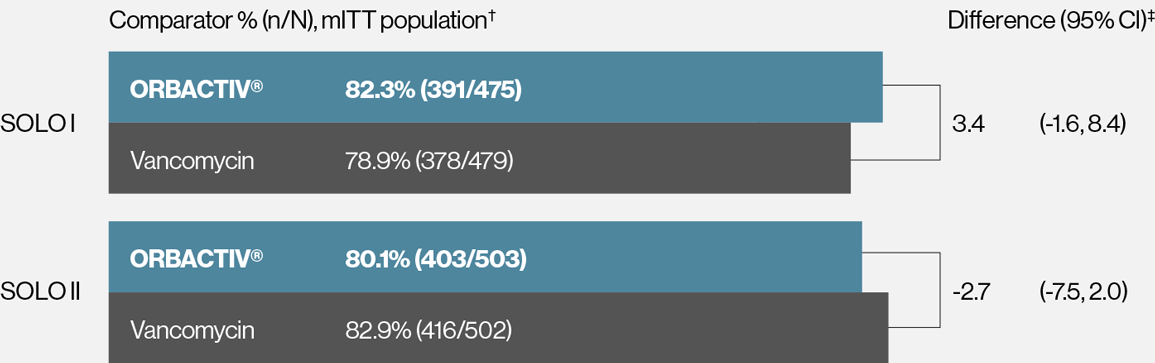
* Early clinical response defined as a composite of the cessation of spread or reduction in size of baseline lesion, absence of fever, and no rescue antibacterial drug at 48-72 hours.
† mITT population: all randomized patients who received any study drug.
‡ 95% confidence interval (CI) based on the normal approximation to binomial distribution.
REFERENCES:
- ORBACTIV® [package insert]: Melinta Therapeutics, Inc.; 2019.
- Corey GR et al. N Engl J Med. 2014;370:2180-2190.
- Corey GR et al. CID. 2014;60(2)254-262.
Single dose ORBACTIV® (oritavancin) was effective at achieving ≥20% lesion size reduction at 48-72 hours1-3
Secondary endpoint: ≥20% lesion size reduction* rates at 48-72 hours
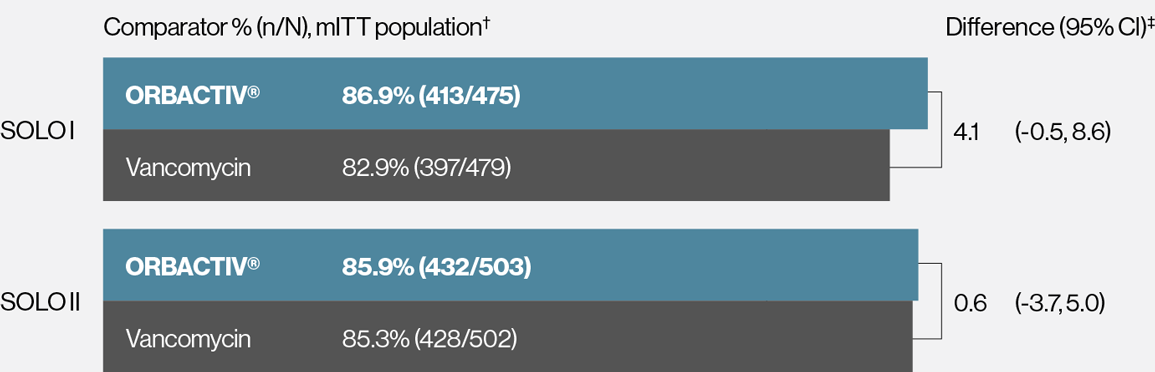
* Patients achieving a 20% or greater reduction in lesion area from baseline at 48-72 hours after initiation of therapy.
† mITT population: all randomized patients who received any study drug.
‡ 95% CI based on the normal approximation to binomial distribution.
REFERENCES:
- ORBACTIV® [package insert]: Melinta Therapeutics, Inc.; 2019.
- Corey GR et al. N Engl J Med. 2014;370:2180-2190.
- Corey GR et al. CID. 2014;60(2)254-262.
Single dose ORBACTIV® (oritavancin) was effective at achieving clinical success at day 14-241-3
Secondary endpoint:Clinical success* rates at day 14-24
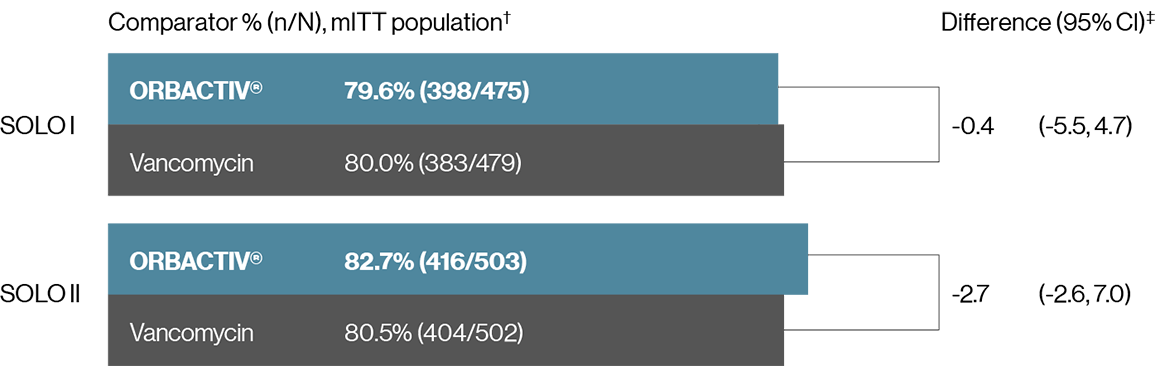
* Investigator-assessed clinical success at day 14–24, defined as complete or nearly complete resolution of baseline signs and symptoms related to primary ABSSSI site such that no further treatment with antibacterial drugs was needed.
† mITT population: all randomized patients who received any study drug.
‡ 95% CI based on the normal approximation to binomial distribution.
REFERENCES:
- ORBACTIV® [package insert]: Melinta Therapeutics, Inc.; 2019.
- Corey GR et al. N Engl J Med. 2014;370:2180-2190.
- Corey GR et al. CID. 2014;60(2)254-262.
Demonstrated consistent effect against designated gram-positive pathogens1
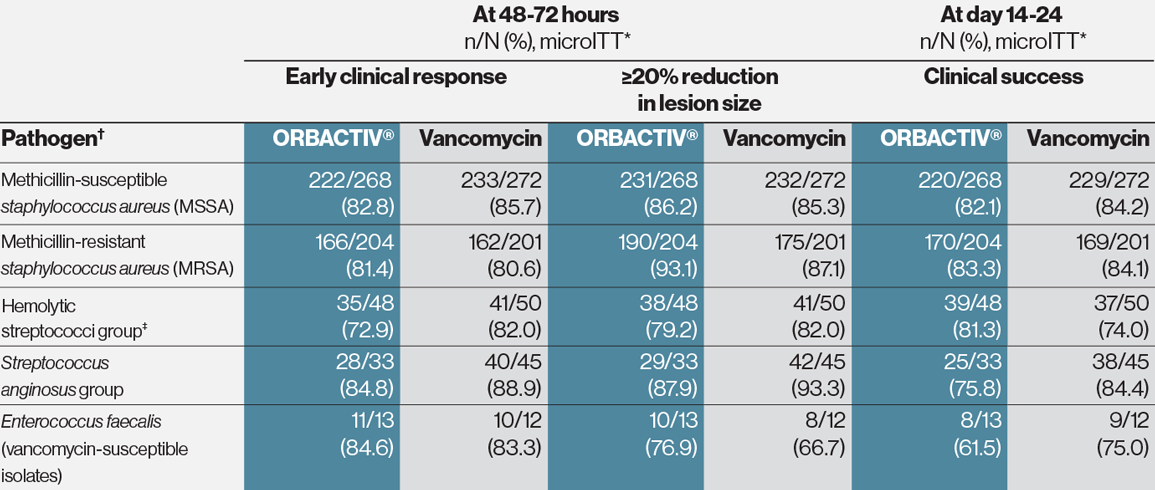
* Microbiological intent-to-treat population.
† Baseline bacteremia in the ORBACTIV® arm with relevant microorganisms causing acute bacterial skin and skin structure infections (ABSSSI) included 4 subjects with MSSA and 7 subjects with MRSA. Eight of these 11 subjects were responders at 48-72 hours after initiation of therapy.
‡ Hemolytic streptococci group includes S. pyogenes, S. agalactiae, and S. dysgalactiae.
REFERENCES:
- ORBACTIV® [package insert]: Melinta Therapeutics, Inc.; 2019.
Single dose ORBACTIV® (oritavancin) demonstrated consistency across endpoints measured in patients with confirmed MRSA1
Response rates for patients with confirmed MRSA in pooled SOLO trials
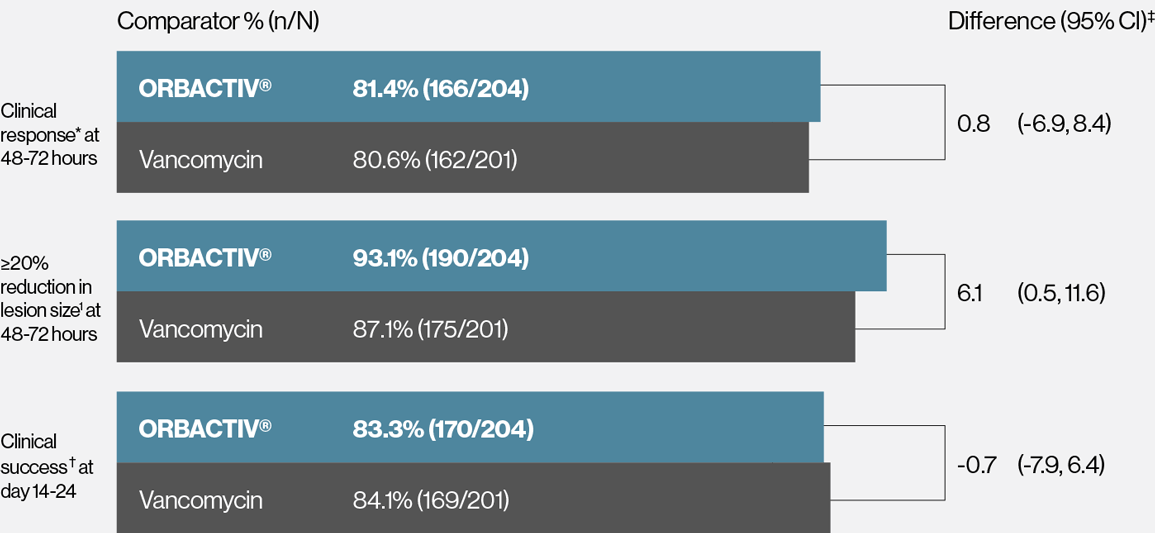
* Early clinical response defined as a composite of the cessation of spread or reductions in size of baseline lesion, absence of fever, and no rescue antibacterial drug at 48-72 hours.
† Patients achieving a 20% greater reduction on lesion area from baseline at 48-72 hours.
‡ Investigator-assessed clinical success at day 14–24, defined as complete or nearly complete resolution of baseline signs and symptoms related to primary ABSSSI site such that no further treatment with antibacterial drugs was needed.
REFERENCES:
- ORBACTIV® [package insert]: Melinta Therapeutics, Inc.; 2019.